https://www.nature.com/articles/s41586-020-2180-5
Sitaatti 26.6.2021. Tässä artikkelissa vertaillaan SARS-2 ja Sars-1 virusten RBD ja RBM kohtia.
- Article
- Published:
Nature volume 581, pages 215–220 (2020)
A new and highly pathogenic coronavirus (severe acute respiratory syndrome coronavirus-2, SARS-CoV-2) caused an outbreak in Wuhan city, Hubei province, China, starting from December 2019 that quickly spread nationwide and to other countries around the world1,2,3. Here, to better understand the initial step of infection at an atomic level, we determined the crystal structure of the receptor-binding domain (RBD) of the spike protein of SARS-CoV-2 bound to the cell receptor ACE2. The overall ACE2-binding mode of the SARS-CoV-2 RBD is nearly identical to that of the SARS-CoV RBD, which also uses ACE2 as the cell receptor4. Structural analysis identified residues in the SARS-CoV-2 RBD that are essential for ACE2 binding, the majority of which either are highly conserved or share similar side chain properties with those in the SARS-CoV RBD. Such similarity in structure and sequence strongly indicate convergent evolution between the SARS-CoV-2 and SARS-CoV RBDs for improved binding to ACE2, although SARS-CoV-2 does not cluster within SARS and SARS-related coronaviruses1,2,3,5. The epitopes of two SARS-CoV antibodies that target the RBD are also analysed for binding to the SARS-CoV-2 RBD, providing insights into the future identification of cross-reactive antibodies.
The emergence of the highly pathogenic coronavirus SARS-CoV-2 in Wuhan and its rapid international spread has posed a serious global public-health emergency1,2,3. Similar to individuals who were infected by pathogenic SARS-CoV in 2003 and Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012, patients infected by SARS-CoV-2 showed a range of symptoms including dry cough, fever, headache, dyspnoea and pneumonia with an estimated mortality rate ranging from 3 to 5%6,7,8. Since the initial outbreak in December of 2019, SARS-CoV-2 has spread throughout China and to more than 80 other countries and areas worldwide. As of 5 March 2020, 80,565 cases in China have been confirmed with the infection and 3,015 infected patients have died (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/). As a result, the epicentre Wuhan and the neighbouring cities have been under lockdown to minimize the continued spread and the WHO (World Health Organization) has announced a Public Health Emergency of International Concern owing to the rapid and global dissemination of SARS-CoV-2.
Phylogenetic analyses of the coronavirus genomes have revealed that SARS-CoV-2 is a member of the Betacoronavirus genus, which includes SARS-CoV, MERS-CoV, bat SARS-related coronaviruses (SARSr-CoV), as well as others identified in humans and diverse animal species1,2,3,5. Bat coronavirus RaTG13 appears to be the closest relative of the SARS-CoV-2, sharing more than 93.1% sequence identity in the spike (S) gene. SARS-CoV and other SARSr-CoVs, however, are distinct from SARS-CoV-2 and share less than 80% sequence identity1.
Coronaviruses use the homotrimeric spike glycoprotein (comprising a S1 subunit and S2 subunit in each spike monomer) on the envelope to bind to their cellular receptors. Such binding triggers a cascade of events that leads to the fusion between cell and viral membranes for cell entry. Previous cryo-electron microscopy studies of the SARS-CoV spike protein and its interaction with the cell receptor ACE2 have shown that receptor binding induces the dissociation of the S1 with ACE2, prompting the S2 to transit from a metastable pre-fusion to a more-stable post-fusion state that is essential for membrane fusion9,10,11,12.
Therefore, binding to the ACE2 receptor is a critical initial step for SARS-CoV to enter into target cells. Recent studies also highlighted the important role of ACE2 in mediating entry of SARS-CoV-21,13,14,15. HeLa cells expressing ACE2 are susceptible to SARS-CoV-2 infection whereas those without ACE2 are not1. In vitro binding measurements also showed that the SARS-CoV-2 RBD binds to ACE2 with an affinity in the low nanomolar range, indicating that the RBD is a key functional component within the S1 subunit that is responsible for binding of SARS-CoV-2 by ACE213,16.
The cryo-electron microscopy structure of the SARS-CoV-2 spike trimer has recently been reported in two independent studies13,17. However, inspection of one available spike structure revealed the incomplete modelling of the RBD, particularly for the receptor-binding motif (RBM) that interacts directly with ACE217. Computer modelling of the interaction between the SARS-CoV-2 RBD and ACE2 has identified some residues that are potentially involved in the interaction; however, the actual residues that mediate the interaction remained unclear18. Furthermore, despite detectable cross-reactive SARS-CoV-2-neutralizing activity of serum or plasma from patients who recovered from SARS-CoV infections15, no isolated SARS-CoV monoclonal antibodies are able to neutralize SARS-CoV-216,17. These findings highlight some of the intrinsic sequence and structure differences between the SARS-CoV and SARS-CoV-2 RBDs.
To elucidate the interaction between the SARS-CoV-2 RBD and ACE2 at a higher resolution, we determined the structure of the SARS-CoV-2 RBD–ACE2 complex using X-ray crystallography. This atomic-level structural information greatly improves our understanding of the interaction between SARS-CoV-2 and susceptible cells, provides a precise target for neutralizing antibodies, and assists the structure-based vaccine design that is urgently needed in the ongoing fight against SARS-CoV-2. Specifically, we expressed the SARS-CoV-2 RBD (residues Arg319–Phe541) (Fig. 1a, b) and the N-terminal peptidase domain of ACE2 (residues Ser19–Asp615) in Hi5 insect cells and purified them by Ni-NTA affinity purification and gel filtration (Extended Data Fig. 1). The structure of the complex was determined by molecular replacement using the SARS-CoV RBD and ACE2 structures as search models4, and refined to a resolution of 2.45 Å with final Rwork and Rfree factors of 19.6% and 23.7%, respectively (Extended Data Fig. 2 and Extended Data Table 1). The final model contains residues Thr333–Gly526 of the SARS-CoV-2 RBD, residues Ser19–Asp615 of the ACE2 N-terminal peptidase domain, one
ion, four N-acetyl-β-glucosaminide (NAG) glycans linked to ACE2 Asn90, Asn322 and Asn546 and to RBD Asn343, as well as 80 water molecules.
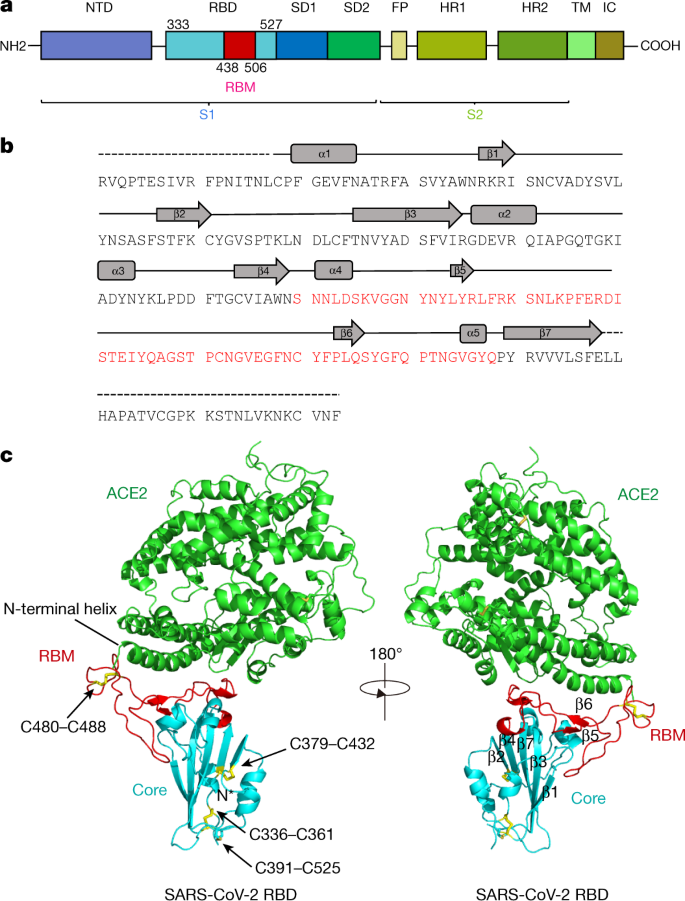
a, Overall topology of the SARS-CoV-2 spike monomer. FP, fusion peptide; HR1, heptad repeat 1; HR2, heptad repeat 2; IC, intracellular domain; NTD, N-terminal domain; SD1, subdomain 1; SD2, subdomain 2; TM, transmembrane region. b, Sequence and secondary structures of SARS-CoV-2 RBD. The RBM sequence is shown in red. c, Overall structure of the SARS-CoV-2 RBD bound to ACE2. ACE2 is shown in green. The SARS-CoV-2 RBD core is shown in cyan and RBM in red. Disulfide bonds in the SARS-CoV-2 RBD are shown as sticks and indicated by arrows. The N-terminal helix of ACE2 responsible for binding is labelled.
The SARS-CoV-2 RBD has a twisted five-stranded antiparallel β sheet (β1, β2, β3, β4 and β7) with short connecting helices and loops that form the core (Fig. 1b, c). Between th
e β4 and β7 strands in the core, there is an extended insertion containing the short β5 and β6 strands, α4 and α5 helices and loops (Fig. 1b, c). This extended insertion is the RBM, which contains most of the contacting residues of SARS-CoV-2 that bind to ACE2. A total of nine cysteine residues are found in the RBD, eight of which form four pairs of disulfide bonds that are resolved in the final model. Among these four pairs, three are in the core (Cys336–Cys361, Cys379–Cys432 and Cys391–Cys525), which help to stabilize the β sheet structure (Fig. 1c); the remaining pair (Cys480–Cys488) connects the loops in the distal end of the RBM (Fig. 1c). The N-terminal peptidase domain of ACE2 has two lobes, forming the peptide substrate binding site between them. The extended RBM in the SARS-CoV-2 RBD contacts the bottom side of the small lobe of ACE2, with a concave outer surface in the RBM that accommodates the N-terminal helix of the ACE2 (Fig. 1c). The overall structure of the SARS-CoV-2 RBD is similar to that of the SARS-CoV RBD (Extended Data Fig. 3a), with a root mean square deviation (r.m.s.d.) of 1.2 Å for 174 aligned Cα atoms. Even in the RBM, which has more sequence variation, the overall structure is also highly similar (r.m.s.d. of 1.3 Å) to the SARS-CoV RBD, with only one obvious conformational change in the distal end (Extended Data Fig. 3a). The overall binding mode of the SARS-CoV-2 RBD to ACE2 is also nearly identical to that observed in the previously determined structure of the SARS-CoV RBD–ACE2 complex4 (Extended Data Fig. 3b).
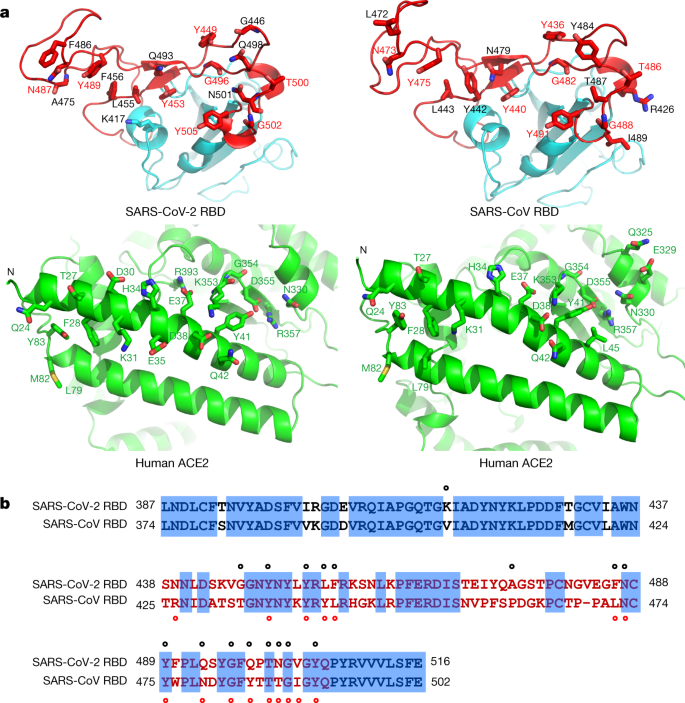
The cradling of the N-terminal helix of ACE2 by the outer surface of the RBM results in a large buried surface of 1,687 Å2 (864 Å2 on the RBD and 823 Å2 on the ACE2) at the SARS-CoV-2 RBD–ACE2 interface. A highly similar buried surface of 1,699 Å2 contributed by SARS-CoV RBD (869 Å2) and ACE2 (830 Å2) is also observed at the SARS-CoV RBD–ACE2 interface. With a distance cut-off of 4 Å, a total of 17 residues of the RBD are in contact with 20 residues of ACE2 (Fig. 2a and Extended Data Table 2). Analysis of the interface between the SARS-CoV RBD and ACE2 revealed a total of 16 residues of the SARS-CoV RBD in contact with 20 residues of ACE2 (Fig. 2a and Extended Data Table 2). Among the 20 ACE2 residues that interact with the two different RBDs, 17 residues are shared between both interactions and most of the contacting residues are located at the N-terminal helix (Fig. 2a and Extended Data Table 2).
a, Contacting residues are shown as sticks at the SARS-CoV-2 RBD–ACE2 and SARS-CoV RBD–ACE2 interfaces. Positions in both RBDs that are involved in ACE2 binding are indicated by red labels. b, Sequence alignment of the SARS-CoV-2 and SARS-CoV RBDs. Contacting residues in the SARS-CoV-2 RBD are indicated by black dots; contacting residues in the SARS-CoV RBD are indicated by red dots.
To compare the ACE2-interacting residues on the SARS-CoV-2 and SARS-CoV RBDs, we used structure-guided sequence alignment and mapped them to their respective sequences (Fig. 2b). Among 14 shared amino acid positions used by both RBMs for the interaction with ACE2, 8 have the identical residues between the two RBDs, including Tyr449/Tyr436, Tyr453/Tyr440, Asn487/Asn473, Tyr489/Tyr475, Gly496/Gly482, Thr500/Thr486, Gly502/Gly488 and Tyr505/Tyr491 of SARS-CoV-2/SARS-CoV, respectively (Fig. 2b). Five positions have residues that have similar biochemical properties despite of having different side chains, including Leu455/Tyr442, Phe456/Leu443, Phe486/Leu472, Gln493/Asn479 and Asn501/Thr487 of SARS-CoV-2/SARS-CoV, respectively (Fig. 2b). The remaining position is at the Gln498/Tyr484 location (Fig. 2b), at which Gln498 of SARS-CoV-2 and Tyr484 of SARS-CoV both interact with Asp38, Tyr41, Gln42, Leu45 and Lys353 of ACE2. Among the six RBD positions with changed residues, SARS-CoV residues Tyr442, Leu472, Asn479 and Thr487 have previously been shown to be essential for binding ACE218. At the Leu455/Tyr442 position, Leu455 of SARS-CoV-2 and Tyr442 of SARS-CoV have similar interactions with Asp30, Lys31 and His34 of ACE2 (Fig. 3a). At the Phe486/Leu472 position, Phe486 of SARS-CoV-2 interacts with Gln24, Leu79, Met82 and Tyr83 of ACE2, whereas Leu472 of SARS-CoV has less interactions with Leu79 and Met82 of ACE2 (Fig. 3a). At the Gln493/Asn479 position, Gln493 of SARS-CoV-2 interacts with Lys31, His34 and Glu35 of ACE2 and forms a hydrogen bond with Glu35; Asn479 of SARS-CoV interacts with only His34 of ACE2 (Fig. 3a). At the Asn501/Thr487 position, both residues have similar interactions with Tyr41, Lys353, Gly354 and Asp355 of ACE2 (Fig. 3a). Asn501 of SARS-CoV-2 and Thr487 of SARS-CoV both form a hydrogen bond with Tyr41 of ACE2 (Fig. 3a). Outside the RBM, there is a unique ACE2-interacting residue (Lys417) in SARS-CoV-2, which forms salt-bridge interactions with Asp30 of ACE2 (Fig. 3b). This position is replaced by a valine in the SARS-CoV RBD that fails to participate in ACE2 binding (Figs. 2b, 3b). Furthermore, a comparison of the surface electrostatic potential also identified a positive charged patch on the SARS-CoV-2 RBD contributed by Lys417 that is absent on the SARS-CoV RBD (Fig. 3b). These subtly different ACE2 interactions may contribute to the difference in binding affinity of the SARS-CoV-2 and SARS-CoV to the ACE2 receptor (4.7 nM compared with 31 nM, respectively) (Extended Data Fig. 4).
Inga kommentarer:
Skicka en kommentar