REVIEW article
The Complement System
The complement system is composed of soluble and cell membrane proteins that regulate the activity of the classical, lectin and alternative pathways (Figure 5). The molecules in these pathways act as sensors to tissue damage and pathogens and as effectors to kill microbes and to clear damaged cells (Reis et al., 2019). In the classical pathway, C1q recognizes pathogens or apoptotic cells directly or indirectly through antibody complexes, or through associations with pentraxins, such as CRP (Sproston and Ashworth, 2018). In the lectin pathway, complement activation is initiated by an interaction involving mannose binding lectin (MBL). Serine proteases [C1r/C1s and MBL-associated serine protease (MASP)] complex with C1q (C1r/C1s) and MBL (MASP). This leads to the cleavage of C4 to its fragments (C4b and C4a) and the formation of a C3 and C5 convertase. In the alternative pathway, C3 is spontaneously hydrolyzed and through the activity of factor D, a C3 convertase is formed, which leads to the formation of a C5 convertase (Ricklin and Lambris, 2007). Additionally, C3 and C5 may also be cleaved by mast cell tryptase, thrombin or kallikrein (Ricklin and Lambris, 2007; Ali, 2010).
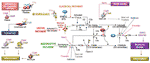
Figure 5 SARS-CoV-2 in the complement system. Classical, lectin, and alternative are the three pathways in the complement system. Complement components C1, C2, C3, and C4 are present in plasma in inactive forms. In the classical pathway, the C1 component, C1q, recognizes apoptotic cells directly or pathogens indirectly through antibody complexes or associations with pentraxins. In the lectin pathway, mannose-binding lectin (MBL) binds the surface of the pathogen. Serine proteases complex with C1q (C1r/C1s) and MBL (MASP: MBL-associated serine protease), which leads to cleavage of C4 to its fragments (C4b and C4a) and the formation of a C3 and C5 convertase. In the alternative pathway, C3 is spontaneously hydrolyzed and through the activity of factor D forms a C3 convertase and subsequently a C5 convertase. Mast cell tryptase, thrombin or kallikrein can also cleave C3 and C5 whereas renin cleaves only C3. Cleavage fragments from these pathways (e.g. C3a, C5a, C5b) activate immune cell subsets to produce inflammation or coagulation. The terminal product of these pathways, C5b6789, is a membrane attack complex (MAC), that creates a pore in cell membranes by displacing phospholipids. The resulting cell lysis induces inflammatory responses. C1q also acts independent of the complement system and binds its receptor (C1qR) on aggregated platelets and endothelial cells in the promotion of coagulation and angiogenesis, respectively. Tissue and organ damage and excessive inflammation in some COVID-19 patients may indicate that SARS-CoV-2 activates the complement cascade. C1, C3, and C5 inhibitors (i) block factor formation in the complement cascade.
Activation of any of these pathways results in the insertion of the membrane attack complex (MAC, composed of C5b–9) into targeted cells and generation of active complement fragments such as C3a, C3b, C4a, C4b, and C5a, which bind complement receptors on a various cell types (Reis et al., 2019) (Figure 5). C3a and C5a are anaphylatoxins and potent stimulators of neutrophils, monocytes, mast cells and platelets, resulting in the release of mediators and the expression of adhesion receptors (Fung et al., 2001; Ali, 2010). Mast cells at rest produce tissue-type plasminogen activator (t-PA) but in the presence of C5a, mast cells generate plasminogen activator inhibitor (PAI)-1 (Wojta et al., 2002). Moreover, C1q also binds a cell surface receptor, C1qR, on aggregated platelets and endothelial cells, resulting in the activation of the coagulation pathway and angiogenesis, respectively (Ghebrehiwet et al., 2019). In viral infections, such as SARS-CoV, PAMPs and DAMPs bind and activate TLRs (Zhao and Zhao, 2020), which regulate the production and function of complement (Hajishengallis and Lambris, 2016). Thus, the innate immune responses involving the complement system are highly implicated in SARS-CoV-2.
SARS-CoV-2 and the Complement System
In COVID-19 patients, elevated plasma CRP levels are a prognostic indicator of adverse outcomes. Threshold values of an adverse outcome have been reported as 27 mg/L (Wang G. et al., 2020) and as 41 mg/L (Luo X. et al., 2020). CRP is primarily synthesized by IL-6-dependent hepatic biosynthesis (Sproston and Ashworth, 2018). In a small study of COVID-19 patients, a combination of IL-6 levels > 80 pg/mL and CRP levels > 97 mg/L were highly predictive of the need for mechanical ventilation (Herold et al., 2020). In human skin fibroblasts, IL-6 induces the production of complement factor B and C3 involved in the activation of the alternative pathway (Katz et al., 1989). Because complement participates in various inflammatory skin diseases (Giang et al., 2018), complement may be produced in response to IL-6 in COVID-19 patients, promoting the cutaneous skin disorders characteristic of COVID-19 (Galvan Casas et al., 2020).
Research involving intranasal infection with recombinant mouse-adapted SARS-CoV (MA15) identified C3 fragments in the lungs of mice one day after infection. Additionally, C3-/- mice exposed to this virus manifested reduced neutrophil and monocyte recruitment and less respiratory dysfunction compared to control mice (Gralinski et al., 2018), demonstrating an active role for the alternative pathway in SARS-CoV. The effects of C3 inhibitors (AMY-101 and APL-9) in COVID-19 subjects with ARDS (ClinicalTrials.gov Identifier: NCT04395456 and NCT04402060) are being tested in clinical trials. Mixed reports involving the binding of the SARS-CoV spike protein to MBL (Leth-Larsen et al., 2007; Zhou et al., 2010), indicate that the function of the lectin pathway in SARS-CoV and SARS-CoV-2 requires further study. Lastly, despite increased production of CRP in SARS-CoV-2 (Wang G. et al., 2020), the functions of the classical pathway have not been systematically explored in SARS-CoV or SARS-CoV-2. Clinical investigations with a C1 inhibitor (Conestat alfa, NCT04414631) and a C5 inhibitor (Zilucoplan, NCT04382755) in severe COVID-19 patients are in progress. Monoclonal antibodies against C5 (e.g. eculizumab, ravulizumab) are also available therapeutics that block excessive complement activation.
In cryoinjured mice, ARB treatment was associated with lower systemic and local levels of C1q, decreased fibrosis and increased myofiber regeneration compared to the controls. The response was reversed by topical C1q and the mechanisms were linked to changes in macrophage C1q production (Yabumoto et al., 2015). Excessive activation of macrophages is associated with the pathophysiology of COVID-19 (Verdoni et al., 2020) and may therefore include macrophage C1q production. Blocking Bruton tyrosine kinase (BTK) is a proposed mechanism to suppress macrophage activation (Roschewski et al., 2020) and is currently being tested in clinical trials of COVID-19 subjects (Acalabrutinib, NCT04380688). Blocking BTK may therefore also affect C1q levels. Moreover, in a rat model of Ang II-induced renal damage, increased circulating levels of complement (C1q, C3, C3c, and C5b-9), CRP, and renal TNF were reduced by a direct renin inhibitor (aliskiren) and also by an ARB (losartan) (Shagdarsuren et al., 2005). The exact roles of Ang II and the SARS-CoV-2 receptor, ACE2, in the complement system require further study.
The increased numbers of apoptotic type I and II pneumocytes and endothelial cells in COVID-19 patient lung tissue (Li et al., 2020) are suggestive of a dysregulated host response in the clearance of these cells. This may involve changes in phagocyte cell surface receptors, the activation state of phagocytes, and/or the response of these cells to components of the complement system (Gordon and Pluddemann, 2018). Neutrophils also undergo apoptosis in inflamed tissue but in ARDS, this process is impaired. In assessing the function of peripheral blood neutrophils from ARDS patients, neutrophils were activated in vitro and produced more NETs and exhibited increased viability compared to healthy control neutrophils. In addition, human monocyte-derived macrophages from the ARDS patients were unable to effectively phagocytose apoptotic neutrophils. However, in the presence of metformin, a 5’ AMP-activated protein kinase (AMPK) activator, the response was improved (Gregoire et al., 2018). Complement component C1q also induces AMPK activation in macrophages (Galvan et al., 2014) and similar to ARDS patients, COVID-19 patient serum exhibits increased NET activity (Zuo et al., 2020). Thus, SARS-CoV-2 may alter the activity or production of C1q, its receptor, the C1 proteases (C1r, C1S), and/or the regulatory crosstalk known to occur between complement and the various TLR ligands (e.g. dsDNA and ssRNA) released from NETs and apoptotic cells (Hajishengallis and Lambris, 2016).
Mast cell-produced tryptase contributes to the cleavage of C3 and C5 (Ali, 2010). Mast cell-produced renin cleaves C3 but not C5 (Bekassy et al., 2018). Because ligands from C3 and C5 cleavage (C3a, C5a) activate mast cells (Ali, 2010) and mast cells may additionally produce ACE (Chao et al., 2011), persistent cross-talk between the complement system and mast cells likely maintains homeostasis in the RAAS and complement pathways. Mast cells also express Mas-related G protein coupled receptor X2 (MRGPRX2) (Ali, 2016). The Ang-(1-7) receptors (Mas, MrgD) and MRGPRX2 are members of a family of ~40 orphan receptors that exhibit ligand promiscuity with AT1R and AT2R in the regulation of the RAAS (Karnik et al., 2017). Moreover, Ang III activates Mas and MRGPRX2 (Gembardt et al., 2008), indicating that Ang III may compete with the ACE2 product, Ang-(1-7), in binding to its receptors (Mas, MrgD, and AT2R) (Figure 4).
The mast cell receptor MRGPRX2 also binds the neuropeptide SP and in response, mast cells produce chemokines (Green et al., 2019). SP, a tachykinin, binds NK1, is released from immune cells and neurons, and enhances inflammatory processes in the lung, gut, and skin (Johnson et al., 2016), which are common sites of inflammation and sources of symptoms in COVID-19 patients (Galvan Casas et al., 2020; Wong et al., 2020). SP and the complement fragments (C3a, C5a) similarly activate mast cells via distinct pathways (el-Lati et al., 1994). SP also acts synergistically with C5a in the recruitment and activation of neutrophils (Perianin et al., 1989). Regulatory cell signals generated by complement fragments and the ligands to Mas-related receptors in mast cells, neutrophils and additional immune subsets may be important to the recruitment of neutrophils and the pathogenesis of various COVID-19 inflammatory disorders.
 Colleen S. Curran
Colleen S. Curran Donna R. Rivera2 and
Donna R. Rivera2 and  Jeffrey B. Kopp
Jeffrey B. Kopp